DynaMesh®-IPOM

DynaMesh®-IPOM implants are designed for soft tissue reinforcement and soft tissue bridging of the fascial and connective tissue structures of the abdominal wall as part of surgical treatment for epigastric hernias, umbilical or incisional hernias, and parastomal hernias following ostomy surgery.
DynaMesh®-IPOMProduct RangeUse and PropertiesVideosDownloadsLiterature
Dual-Layer Composite Mesh
DynaMesh®-IPOM is a dual-component structure specifically developed for the IPOM technique and consists mainly of high-purity PVDF and a small proportion of polypropylene (PP). The parietal side (PP) promotes rapid and safe ingrowth into the abdominal wall. The PVDF layer on the visceral side forms a barrier to the intestines. PVDF demonstrably decreases the risks of adhesions compared with polypropylene [11] and thus reduces the risk of intestinal erosions. If implantation of several meshes is required (for example, the sandwich technique [9]), the open-pore structure means that implants can easily be overlapped.
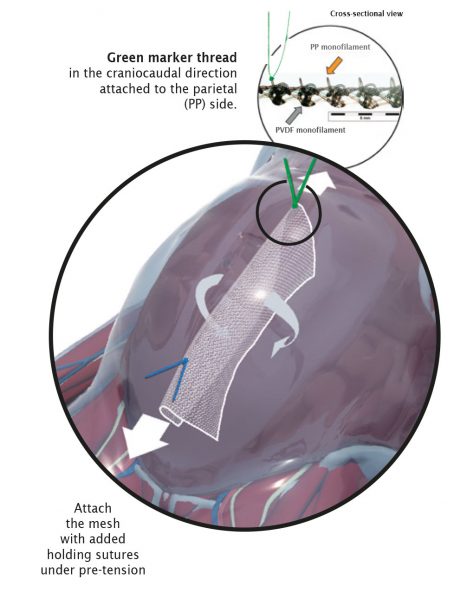
Correct Orientation
The parietal side (PP component) has a green marker thread and must face the abdominal wall. The marker thread is located on the front surface and simultaneously shows the correct direction of the elasticity in the craniocaudal direction.
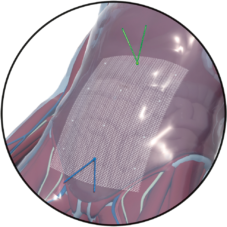
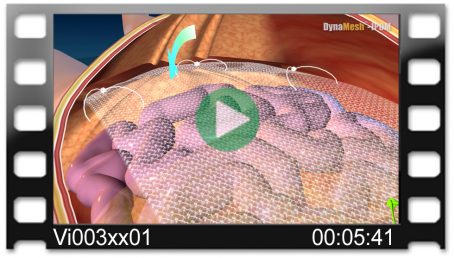
Fold-free mesh positioning after draining the pneumoperitoneum
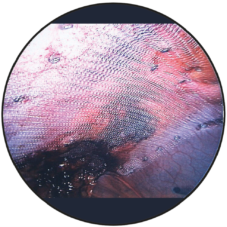
Intraoperative view
Advantages for the Patients
The open-pore mesh construction facilitates the break-down of seroma and reduces scar plate formation.
Minimal mesh shrinkage is achieved and long-term surgical success with high patient comfort is ensured [9-14] through the open-pore and elastic mesh construction made from PVDF, which offers long-term stability.
Abdominal Wall Hernia
Recommended sizes for the surgical treatment of abdominal wall hernias.
When selecting the mesh size, ensure sufficient overlap!
| DynaMesh®-IPOM | d 12 cm round | IP070012F1 | BX = 1 piece | |
| DynaMesh®-IPOM | d 12 cm round | IP070012F3 | BX = 3 pieces | |
| DynaMesh®-IPOM | 10 cm x 15 cm | IP071015F1 | BX = 1 piece | |
| DynaMesh®-IPOM | 10 cm x 15 cm | IP071015F3 | BX = 3 pieces | |
| DynaMesh®-IPOM | 15 cm x 15 cm | IP071515F1 | BX = 1 piece | |
| DynaMesh®-IPOM | 15 cm x 15 cm | IP071515F3 | BX = 3 pieces | |
| DynaMesh®-IPOM | 15 cm x 20 cm | IP071520F1 | BX = 1 piece | |
| DynaMesh®-IPOM | 15 cm x 20 cm | IP071520F3 | BX = 3 pieces | |
| DynaMesh®-IPOM | 20 cm x 20 cm | IP072020F1 | BX = 1 piece | |
| DynaMesh®-IPOM | 20 cm x 25 cm | IP072025F1 | BX = 1 piece | |
| DynaMesh®-IPOM | 20 cm x 30 cm | IP072030F1 | BX = 1 piece | |
| DynaMesh®-IPOM | 20 cm x 30 cm | IP072030F3 | BX = 3 pieces | |
| DynaMesh®-IPOM | 28 cm x 37 cm | IP072837F1 | BX = 1 piece | |
| DynaMesh®-IPOM | 30 cm x 30 cm | IP073030F1 | BX = 1 piece | |
| DynaMesh®-IPOM | 30 cm x 45 cm | IP073045F1 | BX = 1 piece | |
| DynaMesh®-IPOM visible | 30 cm x 30 cm | IP083030F1 | BX = 1 piece | |
Umbilical Hernia
Recommended sizes for the surgical treatment of umbilical hernias.
When selecting the mesh size, ensure sufficient overlap!
| DynaMesh®-IPOM | 07 cm x 06 cm | IP070706F5 | BX = 5 pieces | |
| DynaMesh®-IPOM | |
d 12 cm round | IP070012F1 | BX = 1 piece |
| DynaMesh®-IPOM | |
d 12 cm round | IP070012F3 | BX = 3 pieces |
| DynaMesh®-IPOM | |
10 cm x 15 cm | IP071015F1 | BX = 1 piece |
| DynaMesh®-IPOM | |
10 cm x 15 cm | IP071015F3 | BX = 3 pieces |
| DynaMesh®-IPOM | |
15 cm x 15 cm | IP071515F1 | BX = 1 piece |
| DynaMesh®-IPOM | |
15 cm x 15 cm | IP071515F3 | BX = 3 pieces |
| Product | DynaMesh®-IPOM (1)
DynaMesh®-IPOM visible (2) |
| Field of application | abdominal wall hernia / umbilical hernia |
| Surgical access | laparoscopic / open |
| Surgical technique | IPOM |
| Mesh position | intraperitoneal |
| Fixation | sutures / tacks |
| Green marker thread |
|
| PVDF barrier |
|
| Visible technology |
|
| Dual-component structure |
PVDF monofilament > 85 %
PP monofilament |
| Biocompatibility |
|
| Ageing resistance |
|
| Dynamometry |
|
| Tear propagation resistance |
|
| No scar plate formation |
|
| Classification (Klinge’s classification [8]) | 1a |
![]() Applies to all product sizes
Applies to all product sizes
![]() Does not apply
Does not apply
DynaMesh®-IPOM implants have a parietal side and a visceral side.
The parietal side is identified by green-marked filament ends and consists of PVDF on the surface and a small proportion of PP, whereas the visceral side consists of PVDF on the surface.