DynaMesh®-PRP

DynaMesh®-PRP implants have been specially developed for pelvic floor reconstruction, in laparoscopic or open surgical technique, and serve to support and stabilise fascial structures, connective tissue and ligaments.
The implants are used in the surgical treatment of a prolapse of the vaginal/ cervical stump or uterine prolapse in the pectopexy technique.
DynaMesh®-PRPProduct RangeUse and PropertiesVideosDownloadsLiterature
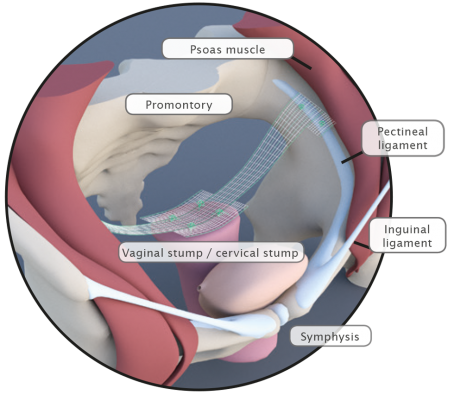
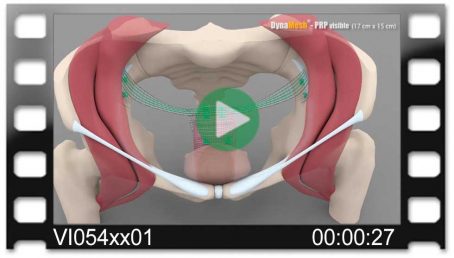
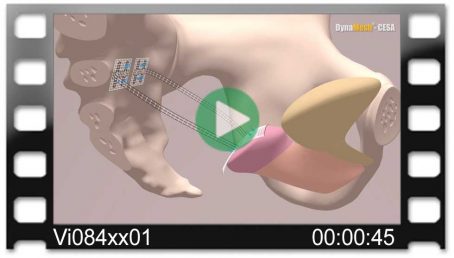
Pectopexy
Bilateral Fixation on the Pectineal Ligament
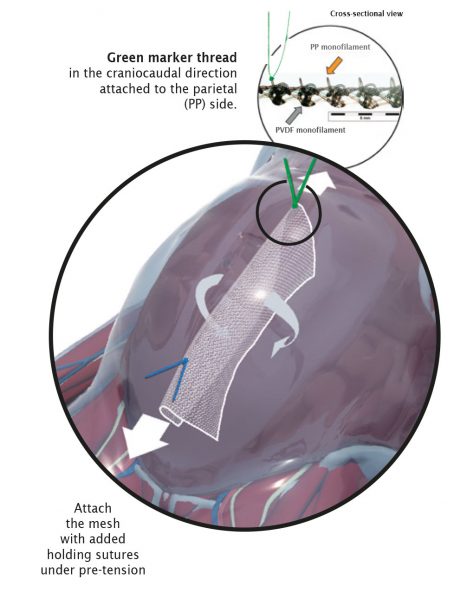
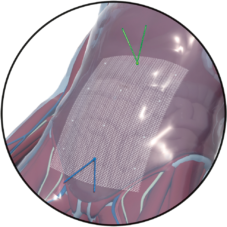
Fig. left:
Apical mesh repair following hysterectomy with DynaMesh®-PRP soft / visible 03 cm x 15 cm

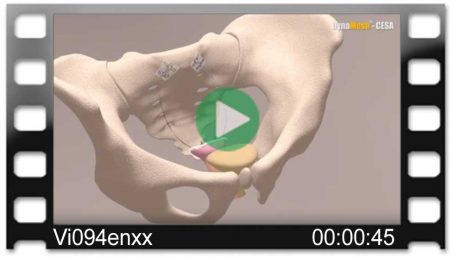
Pectopexy after vaginal/cervical prolapse:
- Two implant sizes are available in the following dimensions DynaMesh®-PRP soft / visible 03 cm x 15 cm and DynaMesh®-PRP visible 03 cm x 18 cm.
- With greatly shortened vaginas, e.g., following a radical hysterectomy, DynaMesh®-PRP visible 03 cm x 18 cm can be optionally used.

Pectopexy after vaginal/cervical prolapse with concomitant cystocele and/or rectocele: (pulsion cystocele / rectocele)
- Additional stabilisation of the affected vaginal wall can be achieved with DynaMesh®-PRP visible 17 cm x 15 cm.

Pectopexy after uterine prolapse with uterine preservation:
- With a normal sized uterus, DynaMesh®-PRP visible 03 cm x 18 cm should be used and fixed in place on the posterior cervix.

Pectopexy after uterine prolapse with uterine preservation:
- With smaller uteri (below 100 g), anterior fixation of DynaMesh®-PRP soft / visible 03 cm x 15 cm can be selected as an alternative.

| DynaMesh®-PRP soft (1) | 03 cm x 15 cm | PV540315F1 | BX = 1 piece |
| DynaMesh®-PRP soft (1) | 03 cm x 15 cm | PV540315F3 | BX = 1 pieces |
| DynaMesh®-PRP visible (2) | 03 cm x 15 cm | PV780315F1 | BX = 1 piece |

| DynaMesh®-PRP visible (3) | 03 cm x 18 cm | PV780318F1 | BX = 1 piece |
| DynaMesh®-PRP visible (3) | 03 cm x 18 cm | PV780318F3 | BX = 3 pieces |

| DynaMesh®-PRP visible (4) | 17 cm x 15 cm | PV781715F1 | BX = 1 piece |
| DynaMesh®-PRP visible (4) | 17 cm x 15 cm | PV781715F3 | BX = 3 pieces |
| Product | DynaMesh®-PRP soft 03 cm x 15 cm (1) DynaMesh®-PRP visible DynaMesh®-PRP visible DynaMesh®-PRP visible |
| Field of application | vaginal/cervical stump or uterine prolapse (1,2)
vaginal stump or uterine prolapse (3) vaginal/cervical stump prolapse, |
| Surgical access | laparoscopic / open |
| Surgical technique | pectopexy bilateral |
| Fixation on vagina / cervix |
sutures
|
| Fixation on pectineal ligament | sutures |
| Specially Warp-knitted Selvedges |
|
| Shape stability |
|
| Defined elasticity |
|
| Visible technology |
|
| Polymer (monofilament) |
PVDF
|
| Biocompatibility | |
| Ageing resistance | |
| Dynamometry | |
| Tear propagation resistance | |
| Classification (Klinge’s classification [8]) | 1a |
![]() Applies to all product sizes
Applies to all product sizes
![]() Does not apply
Does not apply