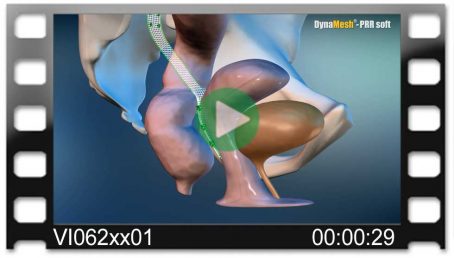
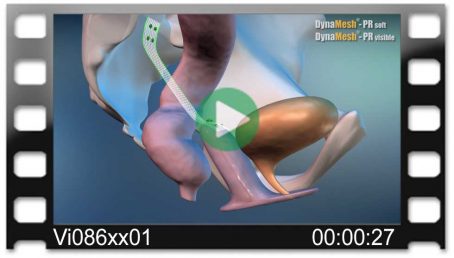
DynaMesh®-PRR

DynaMesh®-PRR implants have been specially developed for pelvic floor reconstruction, in laparoscopic or open surgical technique, and serve to support and stabilise fascial structures, connective tissue and ligaments.
DynaMesh®-PRRProduct RangeUse and PropertiesVideosDownloadsLiterature
DynaMesh®-PRR implants are used in the surgical treatment of the vaginal/cervical stump or uterine prolapse, as well as in the treatment of a concomitant cystocele/rectocele.
Application Examples:

Colpo-/cervicosacropexy
- unilateral
- fixation on vaginal/cervical stump

Colpo-/cervicosacropexy
- unilateral
- fixation on vaginal/cervical stump and anterior mesh plasty for concomitant cystocele

Colpo-/cervicosacropexy
- unilateral
- fixation on vaginal/cervical stump and posterior mesh plasty for concomitant rectocele

Hysterosacropexy
- unilateral
- posterior cervical fixation

Hysterosacropexy
- unilateral
- posterior cervical fixation and posterior mesh plasty for concomitant rectocele
| DynaMesh®-PRR soft |
02/04 cm x 23 cm | PV360423F1 | BX = 1 piece |
| DynaMesh®-PRR soft |
02/04 cm x 23 cm | PV360423F3 | BX = 3 pieces |
| DynaMesh®-PRR visible |
02/04 cm x 23 cm | PV760423F1 | BX = 1 piece |
| Product | DynaMesh®-PRR soft (1)
DynaMesh®-PRR visible (2) |
| Field of application | vaginal/cervical stump or uterine prolapse, concomitant cystocele/rectocele |
| Surgical access | laparoscopic / open |
| Surgical technique | colposacropexy / cervicosacropexy / hysterosacropexy unilateral |
| Fixation on vagina / cervix | sutures |
| Fixation on sacrum | sutures / tacks |
| Specially Warp-knitted Selvedges |
|
| Shape stability |
|
| Defined elasticity |
|
| Visible technology |
|
| Polymer (monofilament) | PVDF |
| Biocompatibility |
|
| Ageing resistance |
|
| Dynamometry |
|
| Tear propagation resistance | |
| Classification (Klinge’s classification [8]) | 1a |
![]() Applies to all product sizes
Applies to all product sizes
![]() Does not apply
Does not apply