DynaMesh®-VASA

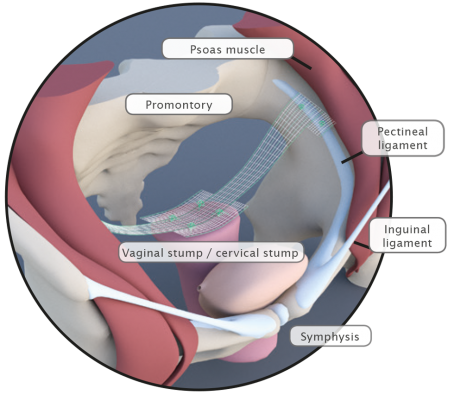
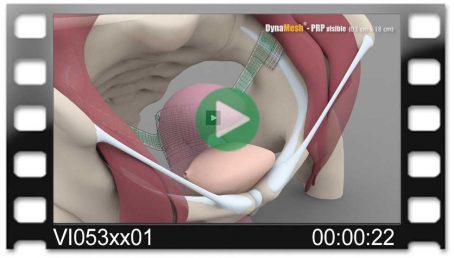
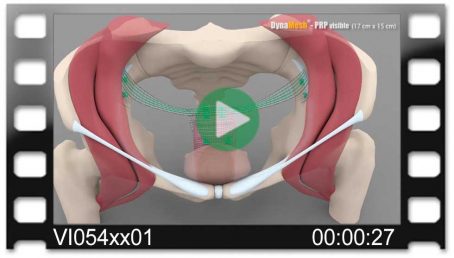
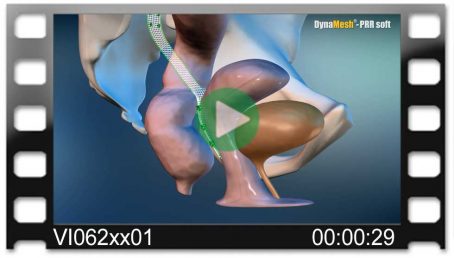
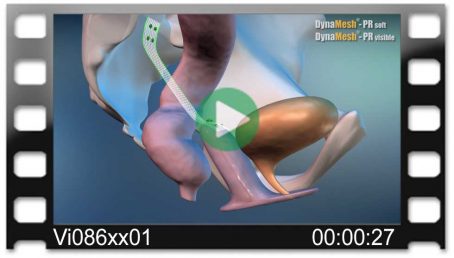
DynaMesh®-VASA implants have been specially developed for pelvic floor reconstruction, and particularly for reinforcing or replacing the uterosacral ligaments, in laparoscopic or open surgical technique.
The implants are used in the treatment of a prolapse of the internal genitalia, such as a vaginal stump prolapse.
DynaMesh®-VASAProduct RangeUse and PropertiesVideosDownloadsLiterature

DynaMesh®-VASA
(VAgino-SAcropexy)
The surgical technique VASA is a modified abdominal colposacropexy procedure (laparoscopic/open), in which the uterosacral ligaments are bilaterally reinforced or replaced by the implant.

DynaMesh®-IVT02 instrument for DynaMesh®-VASA in retroperitoneal tape position through laparotomic access. Reusable instrument made of surgical steel.
Length: 32 cm

- Extraperitoneal tunnelling
- Anatomically adapted to the pelvis
- Eyelet on instrument tip with slanted, atraumatic edges
- Use in laparoscopy
- Reusable instrument

| DynaMesh®-VASA |
02 cm x 03 cm | PV740203F1 | BX = 1 piece |
| DynaMesh®-VASA |
02 cm x 03 cm | PV740203F3 | BX = 3 pieces |
| Product | DynaMesh®-VASA |
| Field of applicationt | vaginal stump prolapse |
| Surgical access | laparoscopic / open |
| Surgical technique | colposacropexy (VASA) bilateral |
| Fixation on vaginal stump |
sutures
|
| Fixation on sacrum |
sutures / tacks
|
| Specially Warp-knitted Selvedges |
|
| Shape stability |
|
| Defined elasticity |
|
| Visible technology |
|
| Polymer (monofilament) |
PVDF
|
| Biocompatibility | |
| Ageing resistance | |
| Dynamometry | |
| Tear propagation resistance | |
| Classification (Klinge’s classification [8]) | 1a |
![]() Applies to all product sizes
Applies to all product sizes