DynaMesh®-SIS direct

DynaMesh®-SIS direct implants are designed as a midurethral sling for soft tissue reinforcement of the pelvic floor as part of the surgical treatment of stress urinary incontinence caused by
a hypermobile urethra and/or intrinsic sphincter deficiency.
DynaMesh®-SIS directProduct RangeUse and PropertiesVideosDownloadsReferencesinternal test-report references

Transobturator (outside-in)

DynaMesh®-SIS direct implants are positioned using the outside-in technique in a transobturator tape position.
Several reusable instruments are available separately to assist the positioning of DynaMesh®-SIS direct implants:

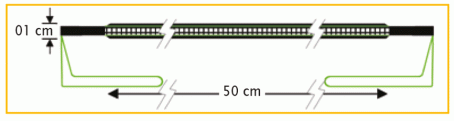
Diameter : 5 -7 cm
DynaMesh®-IST01/-IST02/-IST03:
Instrument set consisting of two instruments (right and left side) for transobturator positioning through transvaginal access using the outside-in technique.

DynaMesh®-IVT01:
Instrument for transobturator positioning through transvaginal access using the outside-in technique.
| DynaMesh®-SIS direct |
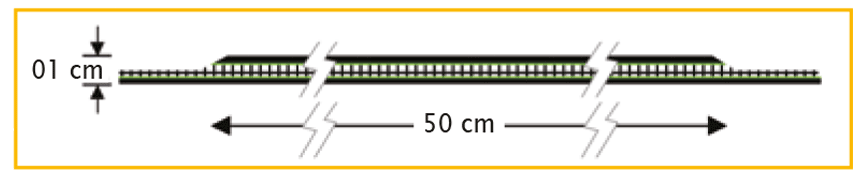
01 cm x 50 cm | PV211050F1 | BX = 1 piece |
| DynaMesh®-SIS direct |
01 cm x 50 cm | PV211050F3 | BX = 3 pieces |
| DynaMesh®-SIS direct soft | 01 cm x 50 cm | PV411050F1 | BX = 1 piece |
| DynaMesh®-SIS direct soft |
01 cm x 50 cm | PV411050F3 | BX = 3 pieces |
| DynaMesh®-SIS direct visible |
01 cm x 50 cm | PV471050F1 | BX = 1 piece |
| DynaMesh®-SIS direct visible |
01 cm x 50 cm | PV471050F3 | BX = 3 pieces |
| Product | DynaMesh®-SIS direct (1)
DynaMesh®-SIS direct soft (2) DynaMesh®-SIS direct visible (3) |
| Field of application | stress urinary incontinence (SUI) |
| Surgical access | transvaginal |
| Surgical technique | TOT – transobturator – outside-in |
| Fixation |
none
|
| Specially Warp-knitted Selvedges |
|
| Shape stability [TR1,TR12] |
|
| Defined elasticity [TR10] |
|
| Visible technology |
|
| Polymer (monofilament) |
PVDF
|
| Biocompatibility [1A,2A,4A,68A,100A,TR1] |
|
| Ageing resistance [101,2A,5B,52B,93A,27A] |
|
| Classification (Klinge’s classification [8]) [TR11] |
1a
|
![]() Applies to all product sizes
Applies to all product sizes
![]() Does not apply
Does not apply
[#] Reference “#” (see “References”)
[#A] Reference “#” (see “References”), “A”: limitation “animal trial”
[#B] Reference “#” (see “References”), “B”: limitation “in-vitro trial”
[TR#] Internal test-report (see “internal test-report references”