DynaMesh®-ENDOLAP

DynaMesh®-ENDOLAP implants serve to support the tissue and stabilise the fascial structures of the groin. They were specially developed for the endoscopic (laparoscopic) repair of inguinal hernias using common minimally invasive surgical techniques (TEP and TAPP).
DynaMesh®-ENDOLAPProduct RangeUse and PropertiesVideosDownloadsLiterature

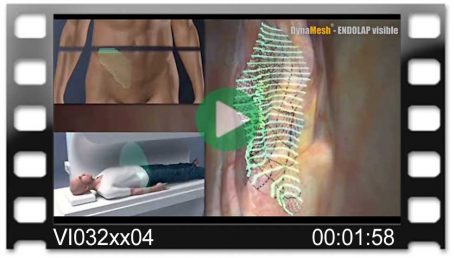
Intraoperative Unfolding
The special textile construction makes it easy to insert the mesh via the trocar and to unfold it intraoperatively. The antislip surface and special selvedges ensure fold-free positioning. The green marker lines perform a dual function. They are used for rapid orientation and visual monitoring of whether the mesh is positioned tension-free.

Choice of Method
DynaMesh®-ENDOLAP was developed specifically for endoscopic (TEP)1) and laparoscopic (TAPP) techniques.
Should the surgeon consider fixation of the implant to be necessary, all fixation methods may be used.
1) Image of surgery courtesy of Dr. A. Kuthe,
DRK-Krankenhaus Clementinenhaus, Hanover, Germany
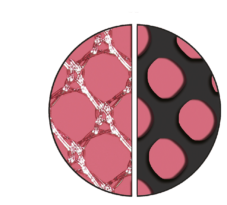
Pore Size
The special warp-knitted structure results in a high textile porosity.
It is the basis for the effective porosity after the formation of the foreign body granuloma, which minimises the risk of scar plate formation.
When selecting the mesh size, ensure sufficient overlap!
| DynaMesh®-ENDOLAP | 10 cm x 15 cm | PV101015F1 | BX = 1 piece |
| DynaMesh®-ENDOLAP | 10 cm x 15 cm | PV101015F3 | BX = 3 pieces |
| DynaMesh®-ENDOLAP | 10 cm x 15 cm | PV101015F10 | BX = 10 pieces |
| DynaMesh®-ENDOLAP | 12 cm x 15 cm | PV101215F3 | BX = 3 pieces |
| DynaMesh®-ENDOLAP | 12 cm x 15 cm | PV101215F10 | BX = 10 pieces |
| DynaMesh®-ENDOLAP | 13 cm x 15 cm | PV101315F3 | BX = 3 pieces |
| DynaMesh®-ENDOLAP | 13 cm x 17 cm | PV101317F3 | BX = 3 pieces |
| DynaMesh®-ENDOLAP | 13 cm x 17 cm | PV101317F10 | BX = 10 pieces |
| DynaMesh®-ENDOLAP | 15 cm x 15 cm | PV101515F3 | BX = 3 pieces |
| DynaMesh®-ENDOLAP | 15 cm x 15 cm | PV101515F10 | BX = 10 pieces |
| DynaMesh®-ENDOLAP visible | 10 cm x 15 cm | PV141015F1 | BX = 1 piece |
| DynaMesh®-ENDOLAP visible | 10 cm x 15 cm | PV141015F10 | BX = 10 pieces |
| Product | DynaMesh®-ENDOLAP (1)
DynaMesh®-ENDOLAP visible (2) |
| Field of application | inguinal hernia |
| Surgical access | endoscopic / laparoscopic |
| Surgical technique | TEP / TAPP |
| Mesh position | preperitoneal (posterior) |
| Fixation | none / sutures / adhesives / tacks |
| Green line marker |
|
| Specially Warp-knitted Selvedges |
|
| Visible technology |
|
| Polymer (monofilament) |
PVDF
|
| Biocompatibility |
|
| Ageing resistance |
|
| Dynamometry |
|
| Tear propagation resistance |
|
| No scar plate formation |
|
| Classification (Klinge’s classification [8]) | 1a |
![]() Applies to all product sizes
Applies to all product sizes
![]() Does not apply
Does not apply