DynaMesh®-HIATUS

DynaMesh®-HIATUS implants are specially developed for prosthetic hiatoplasty and serve to achieve the long-lasting support and stabilisation of the diaphragm in the region of the oesophageal hiatus.
DynaMesh®-HIATUSProduct RangeUse and PropertiesVideosDownloadsLiterature
Shape Stability Under Load
Conventional mesh structures deform under load. Constriction of the mesh in the region of the hiatus may reduce the distance between mesh implant and oesophagus, eventually causing mesh erosion.
DynaMesh®-HIATUS is based on a sophisticated textile design with rectangular pores, which even under load retain a high degree of shape stability.

High Effective Porosity
Mesh implants tend to shrink after incorporation in vivo.
DynaMesh®-HIATUS has a high effective porosity, which ensures that the mesh implant is thoroughly incorporated. During incorporation, the use of the proven and highly biocompatible PVDF polymer ensures that scarring is kept to a minimum. The good incorporation of the mesh implants combined with less scarring leads to minimisation of mesh shrinkage and permanently high flexibility of the incorporated implant.
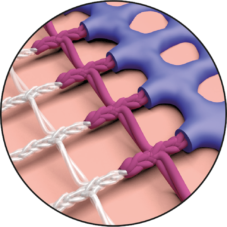
Smooth, Warp-Knitted Selvedges
If the mesh does come into contact with the oesophagus in spite of all measures to prevent it, DynaMesh®-HIATUS has smooth selvedges that can minimise the danger of mesh erosion.

When selecting the mesh size, ensure sufficient overlap!
| DynaMesh®-HIATUS | 07 cm x 12 cm | PV610712F1 | BX = 1 piece |
| DynaMesh®-HIATUS | 07 cm x 12 cm | PV610712F3 | BX = 3 pieces |
| DynaMesh®-HIATUS | 08 cm x 13 cm | PV610813F1 | BX = 1 piece |
| DynaMesh®-HIATUS | 08 cm x 13 cm | PV610813F3 | BX = 3 pieces |
| Product | DynaMesh®-HIATUS |
| Field of application | hiatal hernia |
| Surgical access | laparoscopic |
| Surgical technique | hiatal hernia surgery with implant |
| Fixation | sutures / adhesives / tacks* |
| Shape stability |
|
| Specially Warp-knitted Selvedges |
|
| Visible technology |
|
| Polymer (monofilament) |
PVDF
|
| Biocompatibility |
|
| Ageing resistance |
|
| Dynamometry |
|
| Tear propagation resistance |
|
| No scar plate formation |
|
| Classification (Klinge’s classification [8]) |
1a
|
* Tacks may only be used if an injury to the pericardium can be ruled out with certainty.
![]() Applies to all product sizes
Applies to all product sizes