Male Stress Urinary Incontinence

Application of the implant through perineal access
Transobturator position


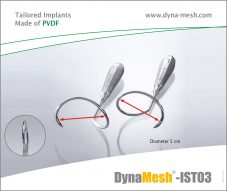
DynaMesh®-IST03
Diameter: 5 cm
DynaMesh®-IST02
Diameter: 7 cm
DynaMesh®-IST03/-IST02:
Instrument set consisting of two instruments (right and left side) for transobturator positioning using the outside-in technique.

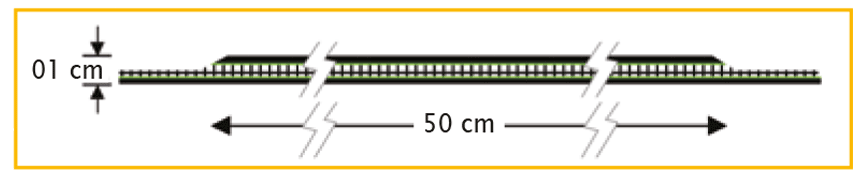
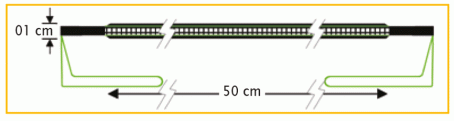
| DynaMesh®-PRM | 04 cm x 03 cm | PV330453F1 | BX = 1 piece |
| DynaMesh®-PRM visible | 04 cm x 03 cm | PV730453F1 | BX = 1 piece |
| Product | DynaMesh®-PRM (1)
DynaMesh®-PRM visible (2) |
| Field of application | stress urinary incontinence |
| Surgical access | perineal |
| Surgical technique | Male Sling TOT – transobturator – outside-in |
| Fixation | synthetic adhesives / sutures |
| Specially Warp-knitted Selvedges |
|
| Shape stability |
|
| Defined elasticity |
|
| Visible technology |
|
| Polymer (monofilament) | PVDF |
| Biocompatibility |
|
| Ageing resistance |
|
| Dynamometry |
|
| Tear propagation resistance | |
| Classification (Klinge’s classification [8]) | 1a |
![]() Applies to all product sizes
Applies to all product sizes
![]() Does not apply
Does not apply